Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Matsuda, Shohei; Yokoyama, Keiichi; Yaita, Tsuyoshi; Kobayashi, Toru; Kaneta, Yui; Simonnet, M.; Sekiguchi, Tetsuhiro; Honda, Mitsunori; Shimojo, Kojiro; Doi, Reisuke; et al.
Science Advances (Internet), 8(20), p.eabn1991_1 - eabn1991_11, 2022/05
Times Cited Count:6 Percentile:58.16(Multidisciplinary Sciences)no abstracts in English
 Am(n,
Am(n, )
)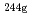 Am and
Am and  Am(n,
Am(n, )
)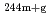 Am reactions
Am reactionsNakamura, Shoji; Shibahara, Yuji*; Endo, Shunsuke; Kimura, Atsushi
Journal of Nuclear Science and Technology, 58(3), p.259 - 277, 2021/03
Times Cited Count:5 Percentile:53.85(Nuclear Science & Technology)Research and development were made for accuracy improvement of neutron capture cross section data on  Am among minor actinides. First, the emission probabilities of decay
Am among minor actinides. First, the emission probabilities of decay  rays were obtained with high accuracy, and the amount of the ground state of
rays were obtained with high accuracy, and the amount of the ground state of  Am produced by reactor neutron irradiation of
Am produced by reactor neutron irradiation of  Am was examined by
Am was examined by  -ray measurement. Next, the total amount of isomer and ground states was examined by
-ray measurement. Next, the total amount of isomer and ground states was examined by  -ray measurement. Thermal-neutron capture cross sections and resonance integrals were derived both for the
-ray measurement. Thermal-neutron capture cross sections and resonance integrals were derived both for the  Am(n,
Am(n, )
)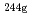 Am and for
Am and for  Am(n,
Am(n, )
)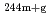 Am reactions.
Am reactions.
Kinoshita, Norikazu*; Nagaoka, Mika; Nakanishi, Takashi*
Science of the Total Environment, 753, p.142087_1 - 142087_10, 2021/01
Times Cited Count:4 Percentile:28.2(Environmental Sciences)The distribution of the anthropogenic radionuclide americium-241 ( Am), a decay product of
Am), a decay product of  Pu discharged from atmospheric tests of nuclear weapons, was investigated to resolve its horizontal and vertical migration in the Tropical East Pacific. We analyzed
Pu discharged from atmospheric tests of nuclear weapons, was investigated to resolve its horizontal and vertical migration in the Tropical East Pacific. We analyzed  Am concentrations in seawater samples collected in 2003. On comparing the
Am concentrations in seawater samples collected in 2003. On comparing the  Am concentrations with the previously determined concentrations of
Am concentrations with the previously determined concentrations of 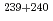 Pu in the same samples, the vertical profiles of
Pu in the same samples, the vertical profiles of  Am were found to be similar to those of
Am were found to be similar to those of 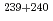 Pu. At some stations, the maximum concentration of
Pu. At some stations, the maximum concentration of  Am occurred 100-200 m deeper than that of
Am occurred 100-200 m deeper than that of 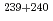 Pu. The
Pu. The  Am/
Am/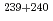 Pu ratios in the North Pacific and South Pacific were comparable to one another, and were typical of the ratio for the Pacific. The
Pu ratios in the North Pacific and South Pacific were comparable to one another, and were typical of the ratio for the Pacific. The  Am distribution was influenced by the water mass at depths below 400 m. The
Am distribution was influenced by the water mass at depths below 400 m. The  Am data support the view there is a current flowing at depths of 400-3000 m from the North Pacific through the equator to the South Pacific. In addition, the
Am data support the view there is a current flowing at depths of 400-3000 m from the North Pacific through the equator to the South Pacific. In addition, the  Am vertical profile was explained by using a box model that considers the decay of
Am vertical profile was explained by using a box model that considers the decay of  Pu and adsorption and scavenging by suspended particles. The different depths for the maximum concentrations of
Pu and adsorption and scavenging by suspended particles. The different depths for the maximum concentrations of  Am and
Am and 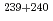 Pu observed at some stations were well explained by the model and by the distribution of CaCO
Pu observed at some stations were well explained by the model and by the distribution of CaCO particles. The residence time of
particles. The residence time of  Am in the Pacific was also estimated by using the model.
Am in the Pacific was also estimated by using the model.
 and (U,Pu,Am,Np)O
and (U,Pu,Am,Np)O
Hirooka, Shun; Matsumoto, Taku; Kato, Masato; Sunaoshi, Takeo*; Uno, Hiroki*; Yamada, Tadahisa*
Journal of Nuclear Materials, 542, p.152424_1 - 152424_9, 2020/12
Times Cited Count:6 Percentile:60.71(Materials Science, Multidisciplinary)The measurement of oxygen potential was conducted at 1,673, 1,773, and 1,873 K for (U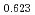 Pu
Pu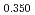 Am
Am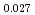 )O
)O and at 1,873 and 1,923 K for (U
and at 1,873 and 1,923 K for (U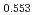 Pu
Pu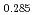 Am
Am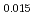 Np
Np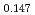 )O
)O by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO
by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.
and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.
 Am(n,
Am(n, )
)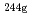 Am,
Am, 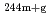 Am reactions
Am reactionsNakamura, Shoji; Endo, Shunsuke; Kimura, Atsushi; Shibahara, Yuji*
KURNS Progress Report 2019, P. 132, 2020/08
Research and development were made for accuracy improvement of neutron capture cross section data on  Am among minor actinides. First, the emission probabilities of decay
Am among minor actinides. First, the emission probabilities of decay  rays were obtained with high accuracy, and the amount of the ground state of
rays were obtained with high accuracy, and the amount of the ground state of  Am produced by reactor neutron irradiation of
Am produced by reactor neutron irradiation of  Am was examinded by
Am was examinded by  -ray measurement. Next, the total amount of isomer and ground states was examoned by
-ray measurement. Next, the total amount of isomer and ground states was examoned by  -ray measurement.
-ray measurement.
Tamain, C.*; Bonato, L.*; Aupiais, J.*; Dumas, T.*; Guillaumont, D.*; Barkleit, A.*; Berthon, C.*; Solari, P. L.*; Ikeda, Atsushi; Guilbard, P.*; et al.
European Journal of Inorganic Chemistry, 2020(14), p.1331 - 1344, 2020/04
Times Cited Count:3 Percentile:23.3(Chemistry, Inorganic & Nuclear)The molecular characterization based on multi-technique approach has led to major highlights on revealing the coordination environment of americium (Am) surrounded by citric acid (H CitH). The structure of the different complexes at pH 1 and 3 are described. These characterizations are made possible by the comparison of the americium-citric acid system with the americium-tricarballylic acid (one analogue of the citric acid without the alpha-hydroxo group).
CitH). The structure of the different complexes at pH 1 and 3 are described. These characterizations are made possible by the comparison of the americium-citric acid system with the americium-tricarballylic acid (one analogue of the citric acid without the alpha-hydroxo group).
 Am neutron capture and total cross sections with ANNRI at J-PARC
Am neutron capture and total cross sections with ANNRI at J-PARCKimura, Atsushi; Nakamura, Shoji; Terada, Kazushi*; Nakao, Taro*; Mizuyama, Kazuhito*; Iwamoto, Nobuyuki; Iwamoto, Osamu; Harada, Hideo; Katabuchi, Tatsuya*; Igashira, Masayuki*; et al.
Journal of Nuclear Science and Technology, 56(6), p.479 - 492, 2019/06
Times Cited Count:14 Percentile:84.54(Nuclear Science & Technology) Am with ANNRI at J-PARC
Am with ANNRI at J-PARCTerada, Kazushi*; Kimura, Atsushi; Nakao, Taro*; Nakamura, Shoji; Mizuyama, Kazuhito*; Iwamoto, Nobuyuki; Iwamoto, Osamu; Harada, Hideo; Katabuchi, Tatsuya*; Igashira, Masayuki*; et al.
Journal of Nuclear Science and Technology, 55(10), p.1198 - 1211, 2018/10
Times Cited Count:18 Percentile:88.27(Nuclear Science & Technology)Nakamura, Shoji; Terada, Kazushi; Takahashi, Yoshiyuki*; Sano, Tadafumi*; Hori, Junichi*
KURRI Progress Report 2015, P. 69, 2016/08
Neutron capture cross section measurements has been conducted for Minor Actinides (MAs) under the research project entitled by "Research and development for Accuracy Improvement of neutron nuclear data on Minor ACtinides (AIMAC)". The present work selected two americium isotopes,  Am and
Am and  Am, were selected, and measurements were carried out by an activation method with neutron sources at KURRI-Linac. It was found that the neutron flux at the target positon was of the order of 10
Am, were selected, and measurements were carried out by an activation method with neutron sources at KURRI-Linac. It was found that the neutron flux at the target positon was of the order of 10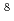 (n/cm
(n/cm s). The reaction rates of
s). The reaction rates of  Am and
Am and  Am were obtained by
Am were obtained by  - and
- and  -ray measurements of the irradiated Am samples.
-ray measurements of the irradiated Am samples.
Tanaka, Kosuke; Sato, Isamu; Hirosawa, Takashi; Kurosaki, Ken*; Muta, Hiroaki*; Yamanaka, Shinsuke*
Journal of Nuclear Science and Technology, 52(10), p.1285 - 1289, 2015/10
Times Cited Count:2 Percentile:17.57(Nuclear Science & Technology)Polycrystalline specimens of americium-containing barium plutonate have been prepared by mixing the appropriate amounts of (Pu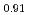 Am
Am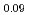 )O
)O and BaCO
and BaCO powders followed by reacting and sintering at 1600 K under the flowing gas atmosphere of dry-air. The sintered specimens had a single phase of orthorhombic perovskite structure and were crack-free. The elastic moduli were determined from the longitudinal and shear sound velocities. The Debye temperature was also determined from the sound velocities and lattice parameter measurements. The thermal conductivity was calculated from the measured density at room temperature, literature values of heat capacity, and thermal diffusivity measured by laser flash method in vacuum. The thermal conductivity of americium-containing barium plutonate was roughly independent of the temperature and was almost the same magnitude as that of BaPuO
powders followed by reacting and sintering at 1600 K under the flowing gas atmosphere of dry-air. The sintered specimens had a single phase of orthorhombic perovskite structure and were crack-free. The elastic moduli were determined from the longitudinal and shear sound velocities. The Debye temperature was also determined from the sound velocities and lattice parameter measurements. The thermal conductivity was calculated from the measured density at room temperature, literature values of heat capacity, and thermal diffusivity measured by laser flash method in vacuum. The thermal conductivity of americium-containing barium plutonate was roughly independent of the temperature and was almost the same magnitude as that of BaPuO and BaUO
and BaUO .
.
Matsumura, Tatsuro; Takeshita, Kenji*
ACS Symposium Series, 933, p.261 - 273, 2006/07
Three TPEN isomers with different positon of nitrogen donor in pyridyl groups, t2pen, t3pen and t4pen, were synthesized and the extraction separation of Am(III) and Eu(III) with these ligands and a fatty acid, decanoic acid, was investigated. All isomers were similar in the complexation in the aqueous phase, such as the protonation and the formation of metal complex, however, they showed different extraction behavior of Am and Eu. The synergistic extraction effect for Am was observed for t2pen and the high separation factor about 100 was measured, when 1:2. The value is comparable to that for the extraction system with a famous nitrogen-donor extractant, BTP. On the other hand, the extractability of other isomers was very low and no separation of Am and Eu was observed. Only t2pen, in which nitrogen donor in pyridyl groups is positioned in the vicinity of the skeletal structure (N-C-C-N structure) of ligand, is available for the extraction separation of Am.
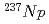 ,
, 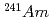 and
and 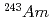
Nakagawa, Tsuneo
Journal of Nuclear Science and Technology, 42(11), p.984 - 993, 2005/11
Times Cited Count:9 Percentile:53.19(Nuclear Science & Technology)no abstracts in English
Mukai, Masayuki; Ueda, Masato; Inada, Daisuke; Yukawa, Kazuhiko; Maeda, Toshikatsu; Iida, Yoshihisa
Proceedings of International Symposium NUCEF 2005, p.219 - 224, 2005/08
For better quantitative understanding of radionuclide migration for safety assessment of geologic disposal, JAERI has been conducting experimental and modeling studies on influences of humic substances, highly alkaline conditions and colloids on sorptive and diffusional behavior of TRU in geologic materials. In the absence of fulvic acid, one of humic substances, diffusion of Am through a tuff sample was not detected. By adding fulvic acid, Am was detected in the downstream cell, which indicates the diffusion through the sample. Highly alkaline conditions arisen from cementitious materials may spread by altering chemical and physical properties of geologic materials. Through-diffusion experiments of alkaline species in granite showed that the effective diffusion coefficient of Ca and OH
and OH in a cement-equilibrated aqueous solution were found to be higher by almost two orders of magnitude than Na
in a cement-equilibrated aqueous solution were found to be higher by almost two orders of magnitude than Na and OH
and OH in a NaOH solution. Radionuclide migration can be enhanced by colloids, and thus a calculation code describing the effect of colloids on radionuclide migration has been required.
in a NaOH solution. Radionuclide migration can be enhanced by colloids, and thus a calculation code describing the effect of colloids on radionuclide migration has been required.
Naganawa, Hirochika; Suzuki, Hideya*; Noro, Junji*; Kimura, Takaumi
Chemical Communications, (23), p.2963 - 2965, 2005/06
A "superweak" anion, TFPB-, gives rise to a field effect on the selectivity for Am over Ln
over Ln in their extraction from aqueous HNO
in their extraction from aqueous HNO solution into benzene containing a "hard donor" extractant that shows no selectivity for these metal ions in traditional solvent extraction.
solution into benzene containing a "hard donor" extractant that shows no selectivity for these metal ions in traditional solvent extraction.
Shimizu, Kaori; Gunji, Kazuhiko*; Haga, Takahisa*; Fukaya, Hiroyuki; Sonoda, Takashi; Sakazume, Yoshinori; Sakai, Yutaka*; Akutsu, Hideyuki; Niitsuma, Yasushi*; Inoue, Takeshi; et al.
JAERI-Tech 2004-078, 27 Pages, 2005/02
Analysis of the uranyl nitrate solution fuel are carried out at the analytical laboratory, NUCEF (the Nuclear Fuel Cycle Engineering Research Facility), which provide essential data for the operations of STACY (the Static Experiment Critical Facility), TRACY (the Transient Experiment Critical Facility) and the fuel treatment system.In the FY 2003, analysis of the uranyl nitrate solution fuel from STACY/TRACY on its pre- and post-operations, analysis of the uranyl nitrate solution under preparation stage for the fuel and analysis for nuclear material accountancy purpose, have been conducted. In addition, analysis on the third U/Pu extraction/separation tests among the preliminary tests to confirm adjustment condition of plutonium solution fuel for its further use at STACY from 2000, and analysis on the experiments to treat extraction waste, were conducted. A total number of analytical samples in the FY 2003 were 156.This report summarizes works related to the analysis and management of the analytical laboratory in the FY 2003.
Yamaguchi, Tetsuji; Nakayama, Shinichi; Yoshida, Takahiro
Radiochimica Acta, 92(9-11), p.677 - 682, 2004/12
Times Cited Count:6 Percentile:40.72(Chemistry, Inorganic & Nuclear)Adsorption of actinides onto negatively charged mineral surfaces was investigated under conditions that actinides were predominantly present as anionic complex species: Th(CO )
)
 , Am(CO
, Am(CO )
)
 , Np(CO
, Np(CO )
) (OH)
(OH)
 , UO
, UO (OH)
(OH)
 , NpO
, NpO (OH)
(OH)
 , Sn(OH)
, Sn(OH)
 and Pb(OH)
and Pb(OH)
 . These solutions were left to stand for 2 days to confirm these elements stable in dissolved state, and then contacted with minerals,
. These solutions were left to stand for 2 days to confirm these elements stable in dissolved state, and then contacted with minerals,  -Al
-Al O
O or SiO
or SiO (AEROSIL, specific surface area: 10
(AEROSIL, specific surface area: 10 m
m kg
kg ). After desired contact time for 2 days or more, the solutions were ultra-filtered through 10
). After desired contact time for 2 days or more, the solutions were ultra-filtered through 10 -molecular-weight cutoff Millipore filters and the concentrations of the elements in the filtrates were determined. The sorption experiments were performed at room temperature (25
-molecular-weight cutoff Millipore filters and the concentrations of the elements in the filtrates were determined. The sorption experiments were performed at room temperature (25 C) under Ar. Distribution coefficients decreased with the increasing pH and with increasing carbonate concentrations. The monotonous decrease in the distribution coefficients in the investigated pH range suggests that the electrostatic repulsion was a dominant interaction between anionic complex species of actinides and negatively charged mineral surfaces.
C) under Ar. Distribution coefficients decreased with the increasing pH and with increasing carbonate concentrations. The monotonous decrease in the distribution coefficients in the investigated pH range suggests that the electrostatic repulsion was a dominant interaction between anionic complex species of actinides and negatively charged mineral surfaces.
Sakai, Yutaka; Gunji, Kazuhiko; Haga, Takahisa*; Fukaya, Hiroyuki; Sonoda, Takashi; Sakazume, Yoshinori; Akutsu, Hideyuki; Niitsuma, Yasushi; Shirahashi, Koichi; Sato, Takeshi
JAERI-Tech 2004-006, 25 Pages, 2004/02
Analyses of the uranyl nitrate solution fuel are carried out at the analytical laboratory, NUCEF (the Nuclear Fuel Cycle Engineering Research Facility), which provide essential data for the operations of STACY (the Static Experiment Critical Facility), TRACY (the Transient Experiment Critical Facility) and the fuel treatment system. In the FY 2002, analyses of the uranyl nitrate solution fuel from STACY/TRACY on its pre- and post-operations, analyses of the uranyl nitrate solution under preparation stage for the fuel and analyses for nuclear material accountancy purpose, have been conducted. In addition, analyses on the preliminary tests to confirm adjustment condition of plutonium solution fuel for its further use at STACY, and analyses on the americium extraction/separation tests to provide americium for the research on high temperature chemistry of TRU, were conducted. A total number of analytical samples in the FY 2002 were 275. This report summarizes works related to the analyses and management of the analytical laboratory in the FY 2002.
Uozumi, Koichi*; Iizuka, Masatoshi*; Kato, Tetsuya*; Inoue, Tadashi*; Shirai, Osamu*; Iwai, Takashi; Arai, Yasuo
Journal of Nuclear Materials, 325(1), p.34 - 43, 2004/02
Times Cited Count:109 Percentile:98.55(Materials Science, Multidisciplinary)Experiments were conducted on simultaneous recovering of uranium and plutonium electrochemically into laboratory scale liquid cadomium cathodes(LCCs) at different U/Pu ratios in the salt phase. The influence of the salt composition on the recovered amount of uranium and plutonium, the morphology of uranium and plutonium in the LCCs, and the behavior of americium were examined. It was shouwn that there is a threshold in the U/Pu ratio in the salt phase for the successful simultaneous recovery of uranium and plutonium up to 10wt% in high current efficiencies.
 Np and
Np and  Am through loess media
Am through loess mediaTanaka, Tadao; Mukai, Masayuki; Maeda, Toshikatsu; Matsumoto, Junko; Ogawa, Hiromichi; Li, Z.*; Wang, X.*; Fan, Z.*; Guo, L.*; Liu, C.*
Journal of Radioanalytical and Nuclear Chemistry, 256(2), p.205 - 211, 2003/05
Times Cited Count:3 Percentile:25.79(Chemistry, Analytical)Migration experiments of  Np(V) and
Np(V) and  Am(III) have been performed using a column system, to investigate migration behavior of Np and Am through a column packed with loess, taken from Shanxi, China. Adsorption mechanisms of Np and Am on the loess were examined by a chemical extraction method. In the case of the Np, most of Np adsorbed on the influent edge of the column. The Np adsorbed on the loess was mainly controlled by surface complexation. However, the migration of Np in the loess media could be roughly evaluated by using the distribution coefficient. In the case of the Am, particulate Am species was formed in the influent solution and moved in the column. The Am adsorbed on the loess was controlled by irreversible reactions. The migration behavior of particulate Am in the loess media could be expressed by the filtration theory.
Am(III) have been performed using a column system, to investigate migration behavior of Np and Am through a column packed with loess, taken from Shanxi, China. Adsorption mechanisms of Np and Am on the loess were examined by a chemical extraction method. In the case of the Np, most of Np adsorbed on the influent edge of the column. The Np adsorbed on the loess was mainly controlled by surface complexation. However, the migration of Np in the loess media could be roughly evaluated by using the distribution coefficient. In the case of the Am, particulate Am species was formed in the influent solution and moved in the column. The Am adsorbed on the loess was controlled by irreversible reactions. The migration behavior of particulate Am in the loess media could be expressed by the filtration theory.
Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Minato, Kazuo; Numata, Masami
Proceedings of GLOBAL2003 Atoms for Prosperity; Updating Eisenhower's Global Vision for Nuclear Energy (CD-ROM), p.2285 - 2291, 2003/00
Stability of AmN and (Am,Zr)N was studied comparatively from the viewpoints of the hydrolytic and evaporative behavior. AmN powder reacted with moisture to form hydroxide Am(OH) , while the solid solution (Am
, while the solid solution (Am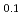 Zr
Zr )N remained stable as long as 1000 hours. Stabilization effect of ZrN was found to depend significantly on its mole fraction from the experiments on (Dy,Zr)N. In the oxidation experiments on (Dy,Zr)N by TG-DTA technique, rapid weight gain by the oxidation occurred above 700 K. Effect of ZrN on the stability against oxygen was slight. Nitrogen release by the evaporation of AmN and (Am
)N remained stable as long as 1000 hours. Stabilization effect of ZrN was found to depend significantly on its mole fraction from the experiments on (Dy,Zr)N. In the oxidation experiments on (Dy,Zr)N by TG-DTA technique, rapid weight gain by the oxidation occurred above 700 K. Effect of ZrN on the stability against oxygen was slight. Nitrogen release by the evaporation of AmN and (Am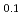 Zr
Zr )N in He gas flow was measured by gas chromatography. Evaporation rate constants of AmN were obtained at 1623-1733 K. Although the evaporation rate constant of AmN in the solid solution were lower than those of the pure AmN, the selective evaporation of AmN from the solid slution were recognized, which resulted in a decrease in the Am mole fraction.
)N in He gas flow was measured by gas chromatography. Evaporation rate constants of AmN were obtained at 1623-1733 K. Although the evaporation rate constant of AmN in the solid solution were lower than those of the pure AmN, the selective evaporation of AmN from the solid slution were recognized, which resulted in a decrease in the Am mole fraction.